There’s plenty of va-va-voom at the bottom!
‘Autonomous fuelled directional rotation about a covalent single bond’ Stefan Borsley, Elisabeth Kreidt, David A. Leigh, Benjamin M. W. Roberts, Nature, 604, 80–85 (2022). . Full Article.
The Nobel Prize-winning physicist Richard Feynman was fascinated by the possibilities of extreme miniaturisation. In his famous lecture ‘There’s Plenty of Room at the Bottom’1 he offered $1000 to ‘the first guy who makes… a rotating electric motor… [that] is only 1/64 inch cube’. He didn’t have to wait long: within a few months Bill McLellan, a mechanical engineer at Electro-Optical Systems, had built and demonstrated such a device (see Video 1). Feynman awarded the prize, but was apparently disappointed that no new scientific developments or discoveries had proved necessary in order to assemble and operate such a small motor.2 McLellan had succeeded using tiny, but conventional, electronics put together with watchmaker’s tools, a fine paintbrush and toothpick!
Fast forward to 9 September 1999 and two related papers appeared back-to-back in the weekly issue of Nature. One was ‘Light-driven monodirectional molecular rotor’3 from Ben Feringa’s group, the first example of a light-driven rotary molecular motor, demonstrating 360° directional rotation about a C=C double bond through photoisomerisation. The other was ‘Unidirectional rotary motion in a molecular system’4 from T. Ross Kelly’s lab. The latter paper described a chemically driven, directional, partial (120°) rotation of a triptycene derivative about a covalent C–C single bond.
Feringa’s paper led to him sharing the 2016 Nobel Prize for Chemistry,5 but Kelly’s aim of continuous chemically-driven 360° rotation about a covalent single bond remained elusive. It is particularly significant as a target because biology’s motors are chemically powered rather than light-driven, often by the biomachine’s catalysis of ATP hydrolysis. Since ‘making is understanding’ creating artificial motors that operate through chemical fuelling can help explain how much more complex motor proteins transduce chemical energy into work through catalysis. This is the subject of considerable contemporary debate and different hypotheses.
In the late-1990s, parallels were drawn by Kelly and others (including an eloquent discussion from Tony Davis6), between the directional rotation about a single bond ‘axle’ and Feynman’s celebrated thought experiment regarding the out-of-equilibrium requirement for directional rotation of a microscopic ‘ratchet-and-pawl’ (Fig. 1).7 Although there were many steps taken towards Kelly’s target in the subsequent 23 years, until now autonomous, chemically fuelled, repetitive 360° rotation about a covalent single bond remained elusive.

Figure 1: Designing brownian ratchet rotary molecular motors. Feynman’s ratchet-and-pawl7 (left): The celebrated thought experiment postulated a molecular-scale ratchet that could extract work from a heat gradient. Kelly’s rotor (middle) was the first step towards realising Feynman’s vision.4 Kelly’s rotor could perform a partial (120°) directional rotation through a sequence of chemical reactions. Now Borsley et al’s motor8 (right) realises the goal of autonomous repetitive 360° directional rotation about a covalent single bond through the presence of a chemical fuel.
Now, the Leigh group have shown that a 26-atom biaryl compound (1-phenylpyrrole 2,2′-dicarboxylic acid) is a catalysis-driven motor that continuously transduces energy from a chemical fuel to induce repetitive 360° directional rotation of the two aromatic rings around the covalent single bond that connects them (Fig. 2).8 Upon treatment of the motor-molecule with a carbodiimide fuel, intramolecular anhydride formation between the rings, and the anhydride’s hydrolysis, both occur incessantly (Video 2). Both the anhydride formation and hydrolysis reactions are directionally biased (they occur faster through particular rotamers of the biaryl system). In this way, catalysis of the hydration of the carbodiimide fuel by the motor continually drives net directional rotation of the rotor around the stator. Each 360° rotation of the motor requires the reaction of a fuel molecule (there is no background ring rotation), takes as little as a few minutes at modest fuel concentrations, and has a directional bias of up to 71:29 with a chirality-matched fuel and additive. In other words, the motor makes a ‘mistake’ in direction every 3–4 turns. Furthermore, because the direction of rotation only depends on the chirality of the fuel and an added hydrolysis catalyst, it is easily reversed.
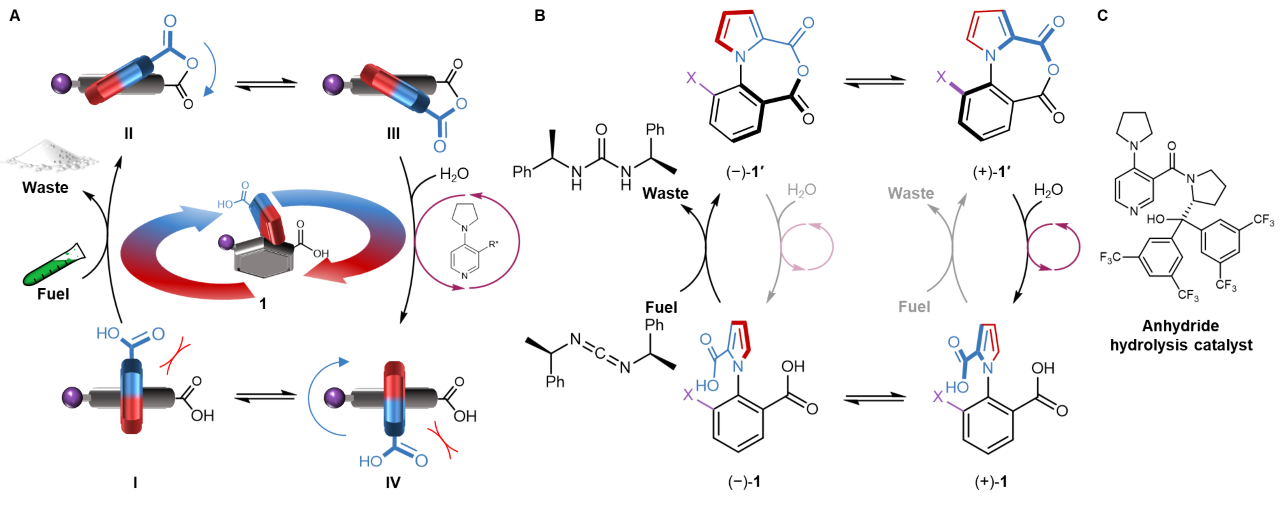
Figure 2: Chemical engine cycle of an autonomous, continuously operating, chemically fuelled single bond rotary motor. (A) Schematic representation of chemically powered rotation of the rotor (pyrrole-2-carbonyl) around the stator (phenyl-2-carbonyl) for 1-arylpyrrole 2,2′-dicarboxylic acid 1.8 Rotation of the rotor carbonyl (blue) past the 6-position (purple) of the stator is favoured in the acid form 1 (IV⇌I), while passing of the rotor carbonyl past the stator carbonyl is only possible in the tethered anhydride form 1′ (II⇌III). The chiral carbodiimide ‘fuel’ kinetically favours anhydride formation I➜II over IV➜III, while the chiral hydrolysis catalyst (shown in part C) kinetically favours III➜IV over II➜I. (B) Chemomechanical cycle for autonomous chemically fuelled rotation of 1. The grey arrows indicate slower transformations than the corresponding black arrows. To be consistent with microscopic reversibility, all transitions should be considered reversible, although under the experimental conditions the position of equilibrium of the fuel-to-waste reaction heavily favours carbodiimide hydration. (C) Chemical structure of anhydride hydrolysis catalyst.
The motor-molecule marks the solution to the long-standing problem of continuous directional rotation about a single covalent bond, and offers insights that should prove useful in developing chemically powered artificial molecular machinery (Fig. 3). Its simplicity should facilitate its optimisation and interfacing with other components for the performance of work and tasks at the molecular level. The mechanism also informs current debates regarding the nature of enzymatic catalysis and biomotor mechanisms.9,10 We hope that Feynman would be pleased :)
Figure 3: How to build a molecular motor. Fuelling cycles, chemical reactivity, stereoelectronics, conformational dynamics, kinetics and thermodynamics all factor into a successful motor-molecule design.
References
1. R. P. Feynman, There’s plenty of room at the bottom. Eng. Sci. 23, 22–36 (1960).
2. P. Ball, Miniature motors. Nat. Mater. 3, 428 (2004)
3. N. Koumura, R. W. J. Zijlstra, R. A. van Delden, N. Harada, B. L. Feringa, Light-driven monodirectional molecular rotor. Nature 401, 152–155 (1999).
4. T. R. Kelly, H. De Silva, R. A. Silva, Unidirectional rotary motion in a molecular system. Nature 401, 150–152 (1999).
5. B. L. Feringa, The Art of Building Small: From Molecular Switches to Motors (Nobel Lecture). Angew. Chem. Int. Ed. 56,11060–11078 (2017).
6. A. P. Davis, Tilting at windmills? The second law survives. Angew. Chem. Int. Ed. 37, 909–910 (1998).
7. R. P. Feynman, R. B. Leighton, M. L. Sands, in The Feynman Lectures on Physics, Vol. 1, Addison-Wesley Publishing Company, Reading, Massachusetts, 1963, Chapter 46.
8. S. Borsley, E. Kreidt, D. A. Leigh, B. M. W. Roberts, Autonomous fuelled directional rotation about a covalent single bond. Nature 604, 80–85 (2022).
9. S. C. Kamerlin, A. Warshel, At the dawn of the 21st century: is dynamics the missing link for understanding enzyme catalysis? Proteins 78, 1339–1375 (2009).
10. B. Ma, R. Nussinov, Enzyme dynamics point to stepwise conformational selection in catalysis. Curr. Opin. Chem. Biol. 14, 652–659 (2010).
