Pumping through Catalysis
‘A catalysis-driven artificial molecular pump’ Shuntaro Amano, Stephen D. P. Fielden and David A. Leigh, Nature, 594, 529-534 (2021). Full Article.
All biomolecular motors are autonomous catalysts;1 they operate continuously as long as unreacted chemical fuel (typically ATP or GTP) is present and stop once all of the fuel is consumed. But this raises two fundamental questions: (1) How can catalysis of a chemical reaction (i.e. acceleration of a chemical transformation by a species that afterwards can be recovered unchanged) cause directional motion of the catalyst? And (2) how does energy released from a catalyzed chemical reaction enable work to be done? Until recently answers to these questions remained elusive. This is because biomotors are so complicated that it is virtual impossible to determine whether the movement of a particular amino acid residue plays a crucial role in the mechanism of the machine or is merely incidental to other, key, conformational or other structural changes.
This situation changed with the invention of the first autonomous chemically-fuelled molecular rotary motor.2 The catenane-based motor (discussed here) continuously rotates the components directionally in the presence of a chemical fuel (Fmoc-Cl) by the (re)attachment of the blocking group supplied by the fuel biasing the random thermal movement of a small ring around a larger track. The catenane is a catalyst for the decomposition of the Fmoc-Cl fuel and, because ‘making is understanding’, provides the first example that explains: (1) how catalysis can lead to directional motion of the components of the catalyst, and (2) how energy released from a catalyzed chemical reaction enables work to be done.
However, other classes of molecular machinery are also powered by molecular engines, including molecular pumps.3 These molecular machines drive substrates energetically uphill, away from thermodynamic equilibrium. Like other biomolecular motors, biological pumps are catalysts, powered by the energy released from their continuous catalytic decomposition of a chemical fuel. So how can catalysis of a chemical reaction cause a substrate to be pumped away from equilibrium? The answer is revealed by the mechanism of operation of the autonomous chemically-fuelled molecular pump shown in Fig. 1.4
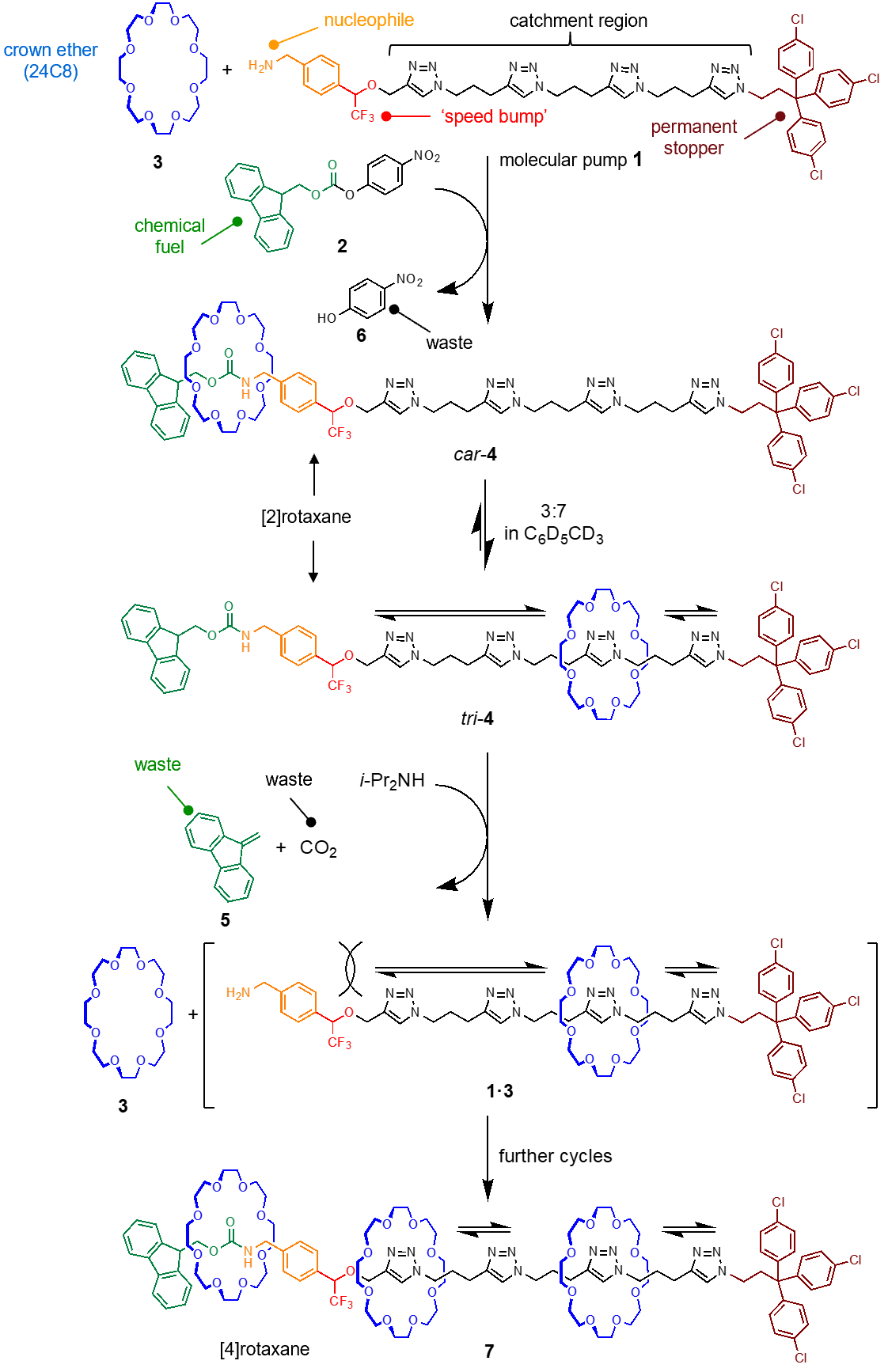
Previous5-8 artificial chemically-driven molecular pumps all required stepwise operations by an external operator; either repeated additions of chemical reagents or the varying of an electric potential during each cycle of pump operation. However, molecule 1, shown in Fig. 1, is an autonomous chemically-fueled molecular pump that, following addition of the fuel, continuously pumps crown ether macrocycles from bulk solution onto a molecular axle without the need for further intervention.4 The pumping is driven by the catalysis of a reaction that transiently inserts a bulky Fmoc ‘stopper’9 at the terminus of the pump to retard the de-threading of captured rings. The result of the pump’s action is dissipatively captured in the form of thermodynamically unstable [n]rotaxanes. The out-of-equilibrium [n]rotaxanes formed (demonstrated with n up to 4) are maintained for as long as unreacted fuel is present, after which the rings slowly de-thread. An animation of the operation of the pump is shown in Video 1.
Pump structure & mechanism of operation
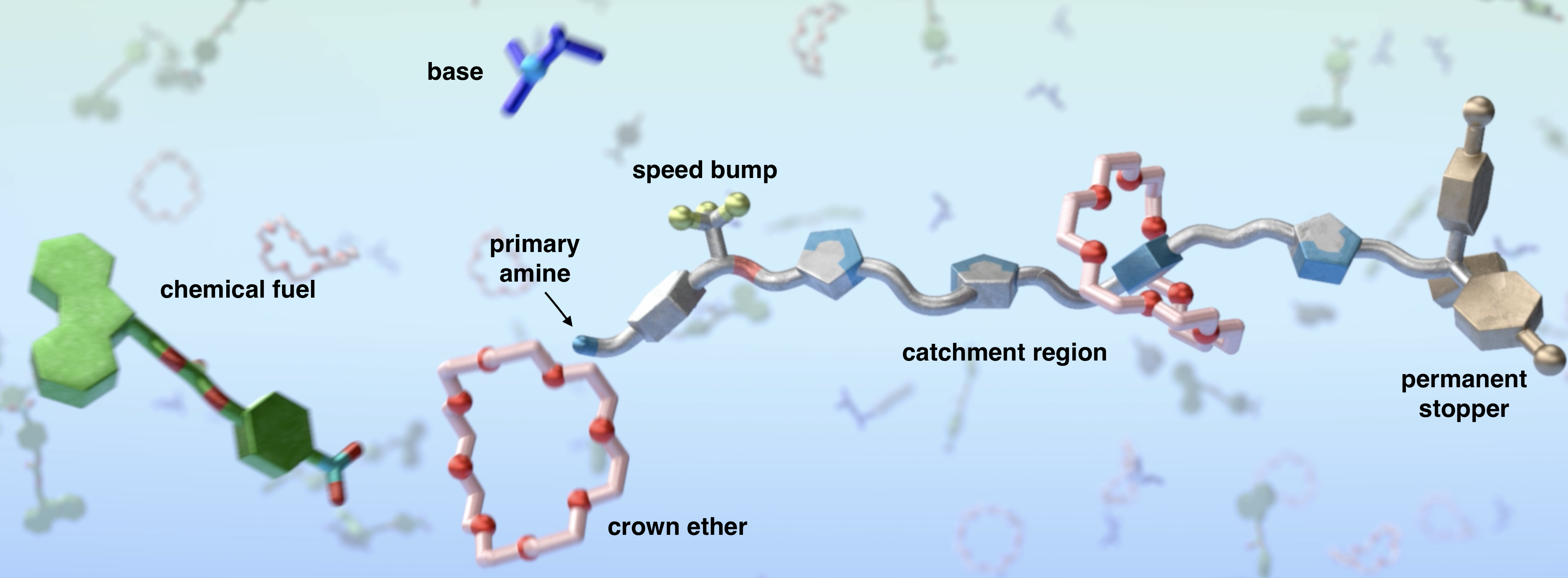
The molecular pump (1, Fig. 1) is a multi-component machine, consisting of several functional parts that collectively contribute to the pump performing the desired function. The pump’s nucleophilic primary amine reacts rapidly with an electrophile (2) threading a crown ether (3) onto the thread by metal-free ‘active template’10 synthesis. A trifluoromethyl (CF3) ‘speed bump’11 means that the captured crown ether only slowly moves from its initial position in the resulting [2]rotaxane 4 (car-4) to the lower energy catchment region featuring triazole rings (tri-4) (Fig.2). A base, diisopropylamine (iPr2NH), present in the reaction mixture slowly decomposes the temporary Fmoc stopper, liberating dibenzofulvene (5) and CO2 (waste) in forming the intermediate kinetically-stable pseudorotaxane 1•3 (Fig. 1). The speed bump retards dethreading of the crown ether in the pseudorotaxane and, in the presence of excess fuel, further fast active template threading reactions occur to generate [3]- and [4]rotaxanes (7; Fig. 1 & Video 1).
Dissipative assembly of [n]rotaxanes
The lowest energy state of the crown ethers is to be unthreaded in bulk solution; at equilibrium the amount of threaded rings on the axle of 1 is vanishingly small. Rotaxane assembly by the pump is dissipative, it proceeds and needs to be maintained by the continuous reaction of the chemical fuel with the pump. Once all of the fuel (2) is consumed, the Fmoc group on the rotaxanes continues to slowly decompose under the basic reaction conditions but can no longer be replaced. With the temporary blocking group gone, the crown ethers dethread, reforming the pump and rings in their original (lowest energy) dethreaded state (Fig. 2 & Video 1).

An important outcome from the development of artificial molecular machine mechanisms is insight into how far more complex biomolecular machines may also operate [‘…perhaps the most important result so far accruing from the synthesis of molecular machines is the insight provided into the fundamental mechanisms by which molecular motors function’, Astumian, R. D. How molecular motors work – insights from the molecular machinist's toolbox: the Nobel Prize in Chemistry 2016. Chem. Sci. 2017, 8, 840]. The use of catalysis, the process used by biological pumps, to drive artificial molecular pumps opens up new opportunities and research directions for the field of catalysis as well as for artificial molecular machinery.
Significance
With the approval of authors and referees, Nature publishes the reports from the peer-review process. The full referee comments are available here but below is a selection of their thoughts regarding the system’s significance:
Referee 1: “The paper, "A catalysis driven artificial molecular pump" by Amano, Fielden, and Leigh describes exciting research that represents a huge step forward in the design and operation of molecular machines. This work is extraordinarily significant in its description of the first autonomous catalysis driven (chemically-fueled) molecular pump. Since 'making is understanding’, the design, synthesis, and operation of the pump shows what part of the minimalist design is responsible for “the what and why” of molecular pumping. This achievement has profound significance not only for constructing synthetic molecular machines but also for our understanding of the mechanisms of much more complex biomolecular pumps and motors.”
Referee 2: “To this reviewer, the molecular machine described in this work is one of the very best reported to date and represents the frontier of the field. The justification of its high value is the combination of a sophisticated operation mechanism (a chemically-driven information ratchet) with a self-assembly operation, demonstrated by the autonomous trapping of multiple macrocycles in a high-energy state. I anticipate that this work will inspire the design of catalysis-driven systems for years, will be used as a model system for the investigation of the principles regulating energy transduction at the molecular level, and will be used as an example in advanced courses on supramolecular chemistry.”
Referee 3: “Biological (machines) pumps use the catalytic decomposition of a chemical fuel to maintain out-of-equilibrium conditions. This manuscript describes the first artificial system to do the same…. The analysis of the data is solid and there are no holes in the arguments. The manuscript is very well written and easy to follow. Indeed, one of the strengths of this work is its (relative) simplicity (read elegance!). The demonstrated use of catalysis to drive artificial molecular machines is game changing and will undoubtedly lead to significant progress in combining catalysis and molecular machinery. This is brilliant work and I recommend publication after attention to one very minor correction.”
We thank the referees for these insights and generous comments.
References
1. Schliwa, M. & Woehlke, G. Molecular motors. Nature 422, 759–765 (2003).
2. Wilson, M. R., Solá, J., Carlone, A., Goldup, S. M., Lebrasseur, N. & Leigh, D. A. An autonomous chemically fuelled small-molecule motor. Nature 534, 235–240 (2016).
3. Qiu, Y., Feng, Y., Guo, Q.-H., Astumian, R. D. & Stoddart, J. F. Pumps through the ages. Chem 6, 1952–1977 (2020).
4. Amano, S., Fielden, S. D. P. & Leigh, D. A. A catalysis-driven artificial molecular pump. Nature 594, 529-534 (2021).
5. Cheng, C., McGonigal, P. R., Schneebeli, S. T., Li, H., Vermeulen, N. A., Ke, C. & Stoddart, J. F. An artificial molecular pump. Nat. Nanotechnol. 10, 547–553 (2015).
6. Erbas-Cakmak, S., Fielden, S. D. P., Karaca, U., Leigh, D. A., McTernan, C. T., Tetlow, D. J. & Wilson, M. R. Rotary and linear molecular motors driven by pulses of a chemical fuel. Science 358, 340–343 (2017).
7. Qiu, Y., Zhang, L., Pezzato, C., Feng, Y., Li, W., Nguyen, M. T., Cheng, C., Shen, D., Guo, Q.-H., Shi, Y., Cai, K., Alsubaie, F. M., Astumian, R. D. & Stoddart, J. F. A molecular dual pump. J. Am. Chem. Soc. 141, 17472–17476 (2019).
8. Qiu, Y., Song, B., Pezzato, C., Shen, D., Liu, W., Zhang, L., Feng, Y., Guo, Q.-H., Cai, K., Li, W., Chen, H., Nguyen, M. T., Shi, Y., Cheng, C., Astumian, R. D., Li, X. & Stoddart, J. F. A precise polyrotaxane synthesizer. Science 368, 1247–1253 (2020).
9. Tian, C., Fielden, S. D. P., Whitehead, G. F. S., Vitorica-Yrezabal, I. J. & Leigh, D. A. Weak functional group interactions revealed through metal-free active template rotaxane synthesis. Nat. Commun. 11, 744 (2020).
10. Fielden, S. D. P., Leigh, D. A., McTernan, C. T., Pérez-Saavedra, B. & Vitorica-Yrezabal, I. J. Spontaneous assembly of rotaxanes from a primary amine, crown ether and electrophile. J. Am. Chem. Soc. 140, 6049–6052 (2018).
11. Baroncini, M., Silvi, S., Venturi, M. & Credi, A. Photoactivated directionally controlled transit of a non-symmetric molecular axle through a macrocycle. Angew. Chem. Int. Ed. 51, 4223–4226 (2012).
